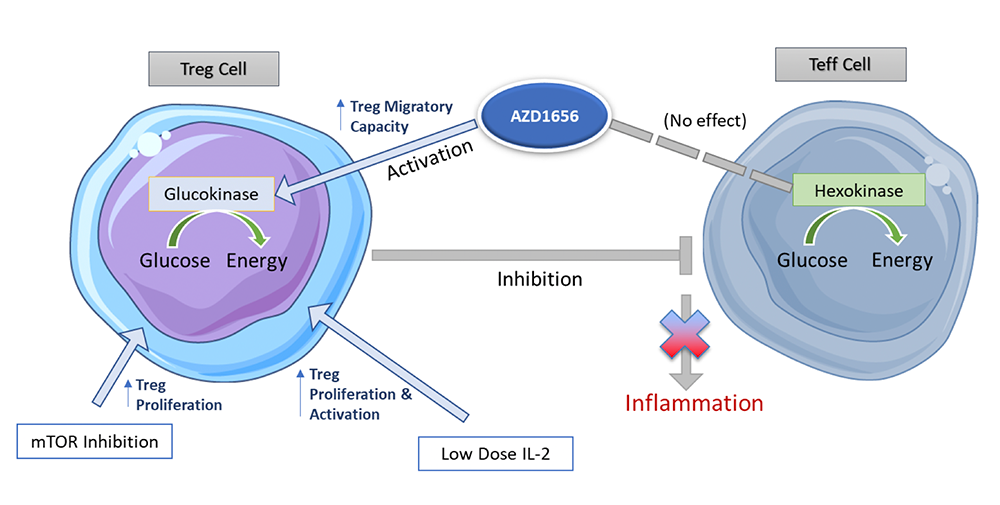
Our current pipeline is focussed on the activation of Tregs using glucokinase activator AZD1656 to treat inflammatory-driven disease.
- AZD1656 is a specific glucokinase agonist – originally developed for type 2 diabetes. Over 1000 people have been dosed with AZD1656 during development with no significant side effects
- A novel scientific insight emerged from QMUL that T regulatory cells specifically use glucokinase to power migration. This led to the realisation that AZD1656 could treat many autoimmune diseases by reducing inflammation using “in vivo cell therapy” via a small molecule activator
Current programmes
- Leishmaniasis is traditionally thought of as a neglected tropical disease but much of Southern Europe is considered highly endemic for Leishmaniasis. Salt Hill are investigating the use of AZD1656 in treating Cutaneous Leishmaniasis using a preclinical model in Brazil. This study began in May 2025 and is due to report in August 2025.
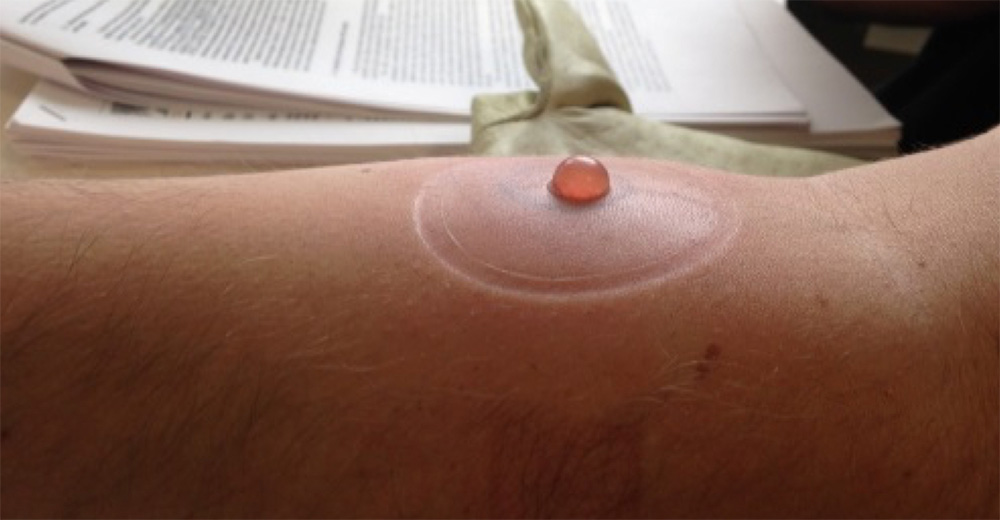
- Gilroy blister study – a mechanistic study in 42 volunteers to investigate the use of AZD1656 in a skin blister model of inflammation. Will inform dose and future disease selection. Interim results in August 2025.
Planned programmes
- Pathfinder studies planned in 3 diseases with chronic unmet need:
- Graves’ disease – a prevalent autoimmune disorder that affects the thyroid gland
- Membranous Nephritis – an autoimmune disease that affects the glomeruli in the kidneys
- Minimal Change Glomerulonephritis – an autoimmune condition that affects the podocytes of the kidneys, particularly found in children
